That’s a lot of words but, simply put, MOST offers suspensions that find applications as catalysts in photocatalytic oxidation (PCO) to treat or remove chemical contaminants in feed streams of air. We can also consult with you on developments related to PCO, just contact us to talk about your product or project.
Activation
Photocatalytic oxidation (PCO) catalysts are activated when they are illuminated with light that has sufficient energy (i.e., low enough wavelength) to excite an electron from the valence band to the conduction band of the catalyst (i.e., across the material’s band gap).
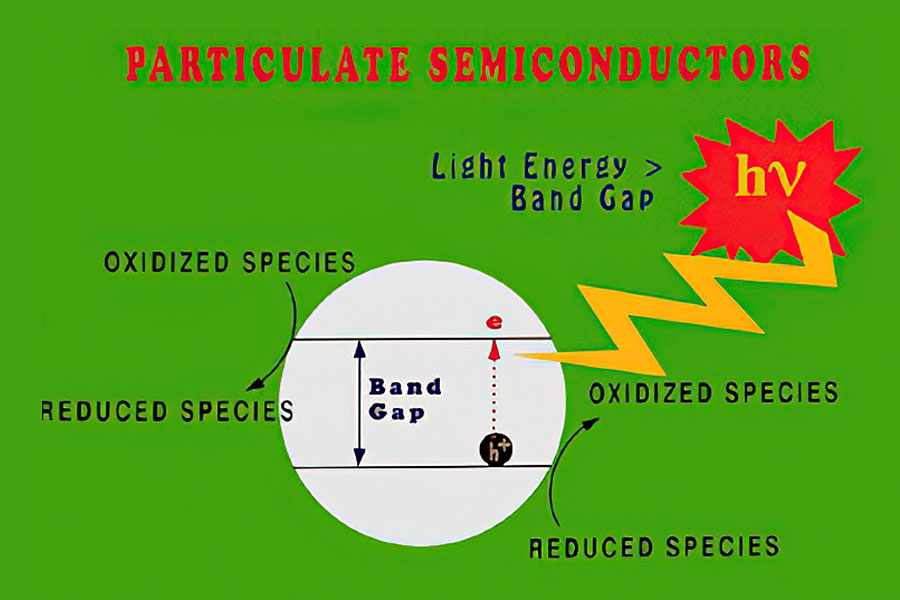
Most commercial PCO and electrically enhanced PCO systems use titania-based photocatalysts, in large part because these materials maintain their activity unless they are poisoned by other species present in the feed stream or by those generated during the photooxidation process. MOST offers a pure titania sol for electrically enhanced PCO and some PCO applications and a mixed zirconia-titania sol for PCO applications as it has not proven effective for electrically enhanced PCO. Keep in mind, titania-based photocatalysts require near-UV light (< 380 nm) for activation.

Adherence to Substrates
There are a number of design factors in producing an active photocatalytic material. We are well versed in all of them. Please contact us for materials to achieve your goals.
UW Research

Previously, PCO reactors from other commercial suppliers had been tested at the site. As an alternative, workers at the site successfully tested a suitcase-sized prototype PCO reactor fabricated by the UW-Madison researchers (i.e., MOST co-founders).
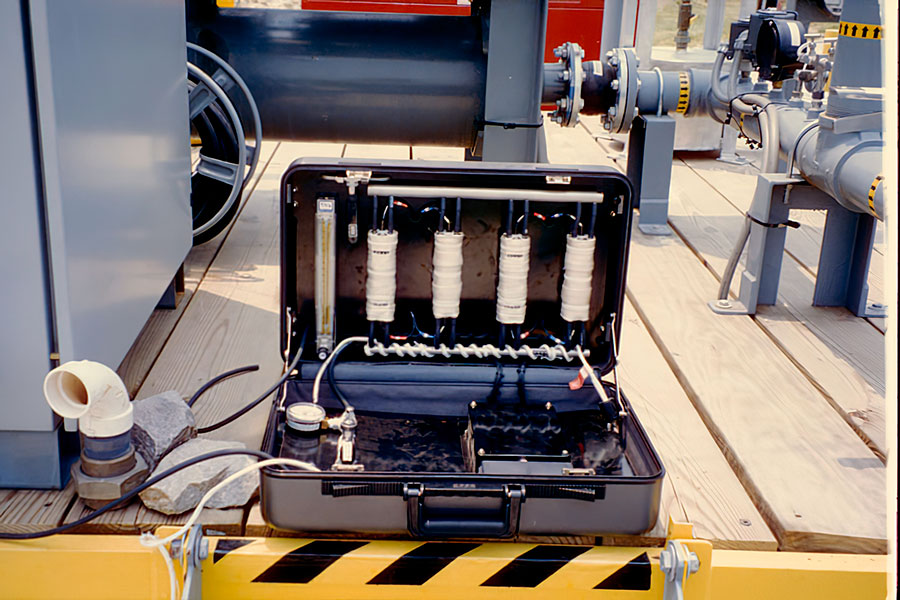
Our Prototype Reactor
This PCO reactor contained four annular glass reactors wrapped in white heat tape and mounted vertically during operation. Each annular reactor contained a UV-fluorescent bulb down the center of the annulus. Each reactor was filled with titania photocatalyst.
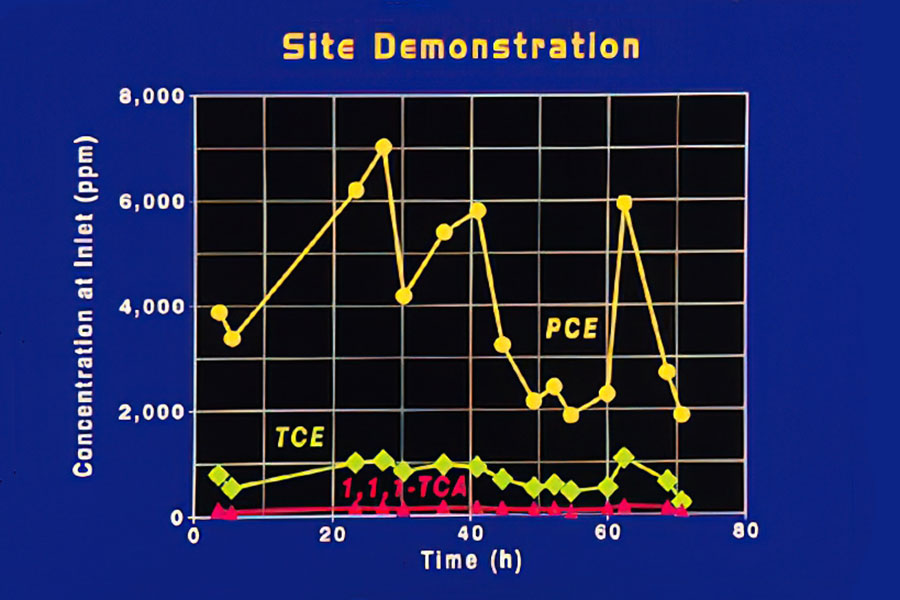
Inlet Concentrations
This figure shows the inlet concentrations of three contaminants in the offgas from the groundwater at the Savannah River site over a 3-day period. Concentration variations were influenced by temperature and time of day.
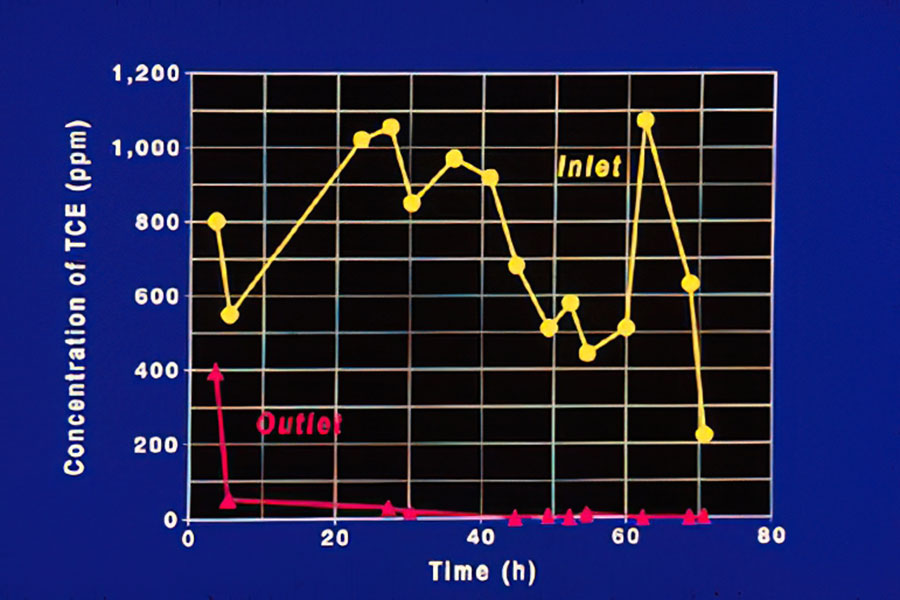
Concentration of TCE
This figure shows the destruction of a small stream of offgas containing TCE over a 3-day period.
Photocatalysts Coated on Glass
This experimental setup was used to test the performance of titania-based photocatalysts coated on glass. The bluish color is from a UV-fluorescent bulb placed down the center of an annular reactor whose annulus contained coated glass beads.
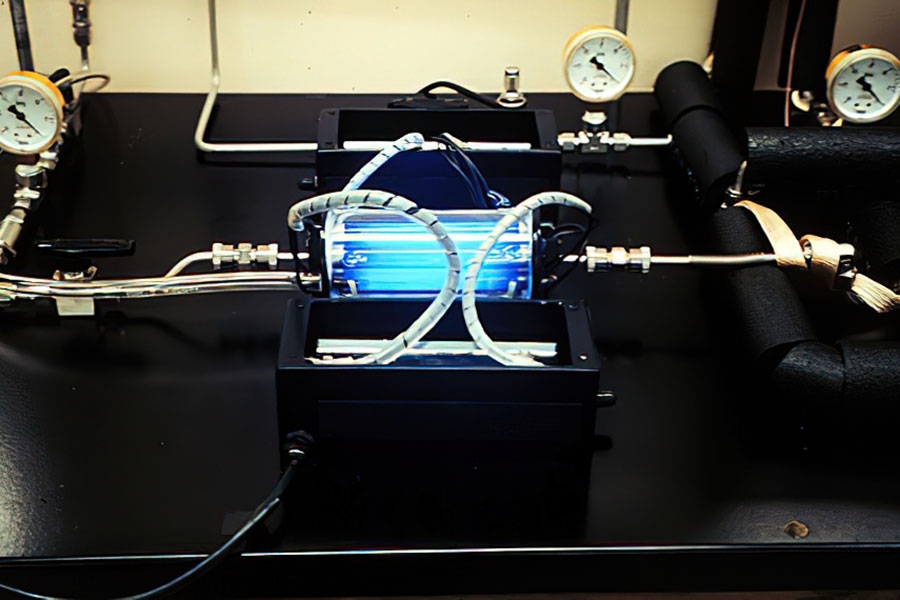
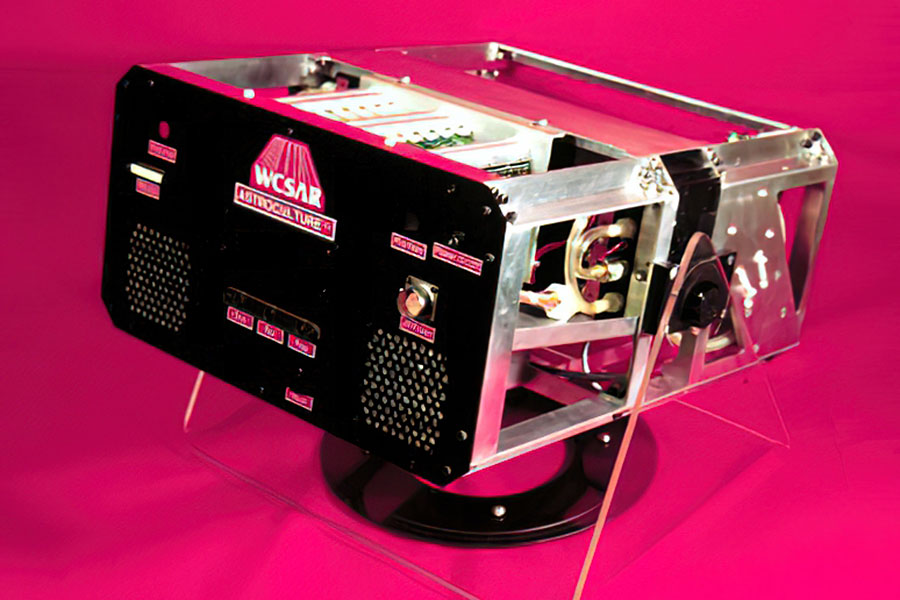
Ethylene Removal … on the Space Shuttle
This is a prototype photoreactor fabricated and tested at the Wisconsin Center for Space Automation and Robotics (WCSAR) at the UW-Madison (using materials identical to those fabricated by MOST). This reactor flew on multiple Space Shuttle missions for the purpose of removing ethylene gas to allow proper plant growth in space.
